An emulsifier is the best mixing element that allows oil and water to mix. Detergent is one of the reference examples of emulsions.
Mixing Oil and Water
Do you know something?
Whatever magic you do, few elements never become friends with each other. Oil and water are the best examples. Try to mix them, and these will never go with each other. Or do they mix? You can try out this experiment on your own.
For this, you will need Water, Food coloring, a small Soft Drink bottle, Dishwashing liquid or detergent, and two tablespoons of cooking oil.
Make sure to follow the below Instructions:
- Add one or two drops of food coloring into the water in a cup.
- Take a small Soft Drink bottle, add about two tablespoons of the colored water and two tablespoons of Cooking oil.
- Close the lid and shake the bottle as much as you can.
- Keep the bottle down and check what happens. You can find the elements are mixing, and the oil will be floating on the top.
- Now add a pinch of detergent and try shaking the bottle. Notice the changes.


Why don’t oil and water mix?
Water molecules are polar molecules. That means one end of the molecule has a positive, and the other end has a negative charge, which allows, water molecules to bind together. Oil molecules are non-polar – they have no charge because of this, these are more attracted to each other than to water molecules.
Water molecules are more attracted to each other than to oil molecules.
Emulsions are a mixture of two immiscible substances.
Emulsifiers are additives that help two immiscible liquids mix as Casein is the emulsifying agent in milk that keeps two immiscible layers of fat and water together. Soap acts as a cleaning agent being an emulsifier, Egg Yoke is used in making Cookies and Cakes as it acts as an emulsifier mixing butter, oil, and water.
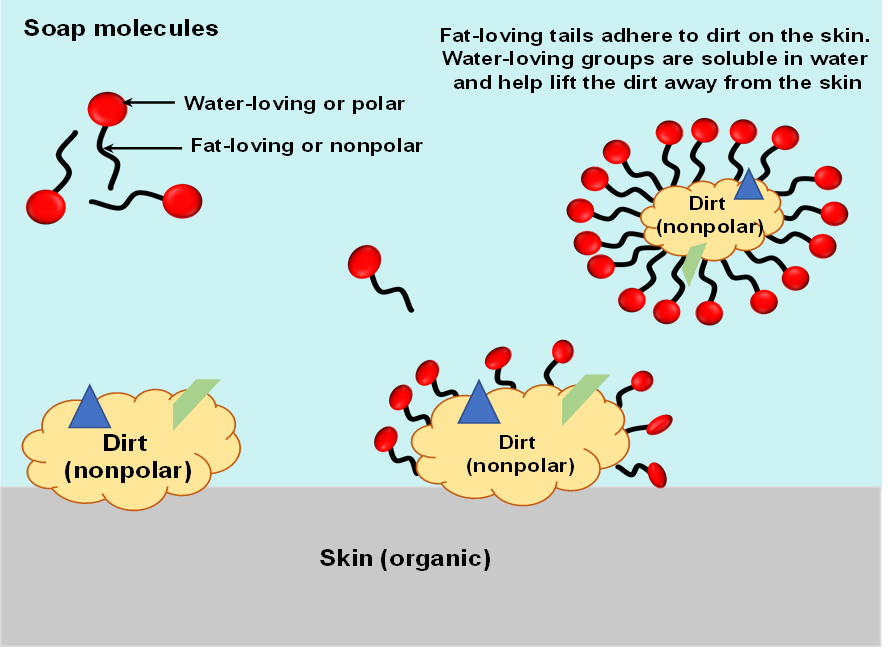
Why soap and detergent are magical
Soap cleans oil and grease because one end of the soap molecule is polar and soluble in water, and the other end is non-polar and similar to oil and grease. The soap molecules surround grease by leaving the water-soluble parts on the outside so the copy rolex water can help wash the wax away. Thus, the soap molecule provides a link between two substances(oil and water) that would otherwise be immiscible(non-soluble).